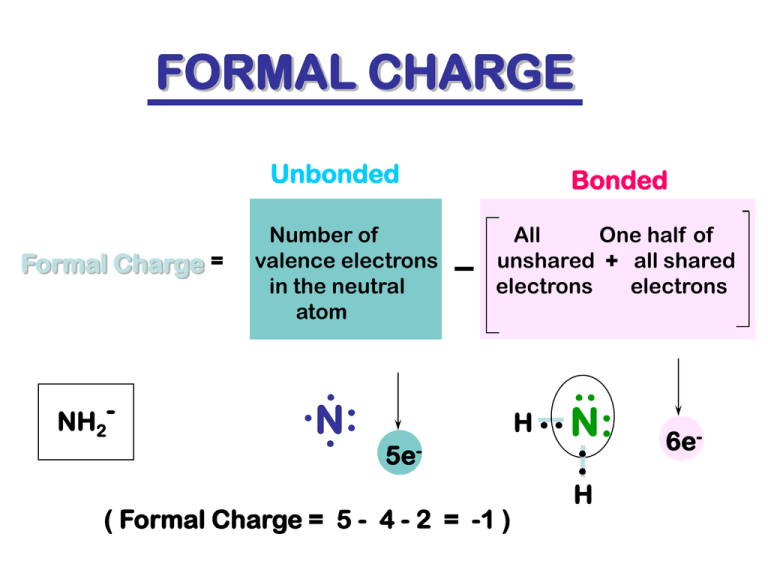
FORMAL CHARGE
Bonding electrons = 1 single bond = 2 electrons. Non-bonding electrons = 3 lone pairs = 3 (2) = 6 electrons. Formal charge on the single bonded Oxygen atom = 6 - 6 - 2/2 = 6 - 6 - 1 = 6 -7 = -1. ∴ The formal charge on the single-bonded O-atom in O3 is -1. This calculation shows that zero formal charges are present on double-bonded O.
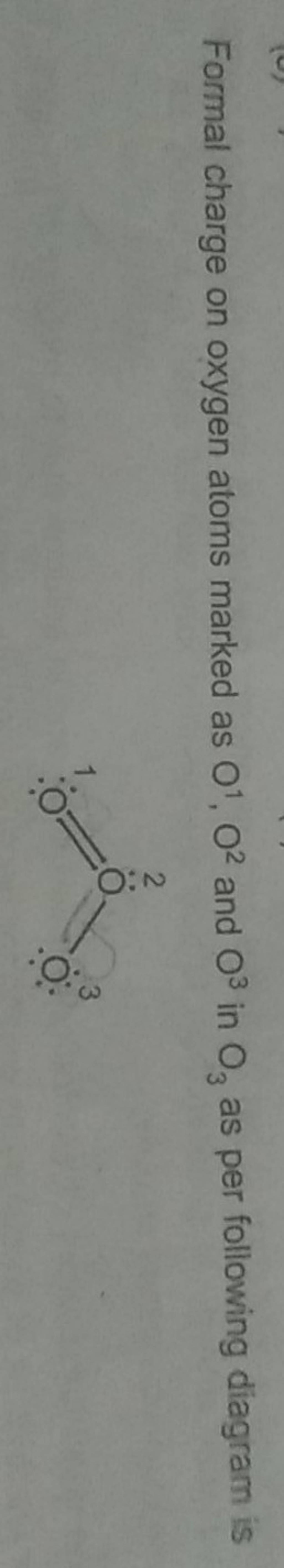
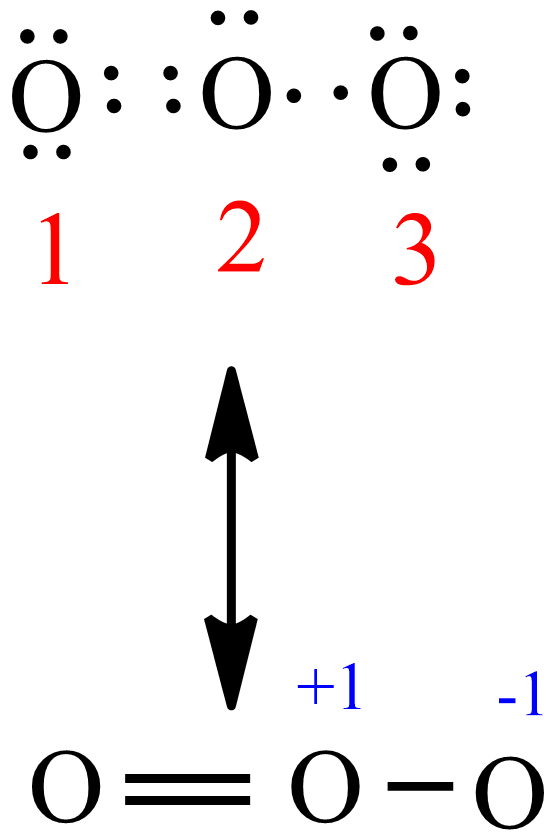
Formal charge on oxygen atoms marked as O1,O2 and O3 in O3 as per follow..
Explanation: Simple VESPER requires that we distribute 3 ×6 = 18 valence electrons across 3 centres: O = O+ − O−. From the left, O1, has TWO lone pairs; O2 has ONE lone pairs; and O3 has THREE lone pairs. And thus the formal charge of each oxygen atom ( 8e−,7e−,9e−) is 0, + 1, −1 respectively.

[Expert Answer] the the formal charges of oxygen labelled 321 in oxygen respectively are
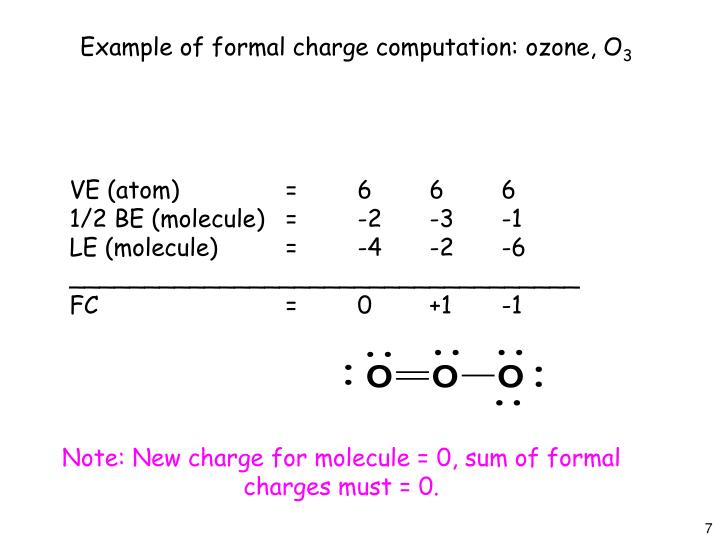
Explanation: A formal charge is equal to the number of valence electrons of an atom MINUS the number of electrons assigned to an atom. Consider the resonance structures for O3. Oxygen has 6 valence electrons. Look at the top left oxygen atom. It has two lone pairs ( 4 electrons) and a double bond ( 2 electrons).

Formal Charge 34. The formal charges on the three oxygen atoms in O3 mol..
Formal charge in O 3 ( Ozone): In an O 3 molecule, the formal charge on the middle oxygen atom ( 2) is + 1. In an O 3 molecule, the formal charge on the left oxygen atom ( 3) is - 1. In an O 3 molecule, the formal charge on the right oxygen atom ( 1) is 0.

Calculate formal charge of central atom O3 O=[Os] Filo
In order to calculate the formal charges for O3 we'll use the equationFormal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding electr.
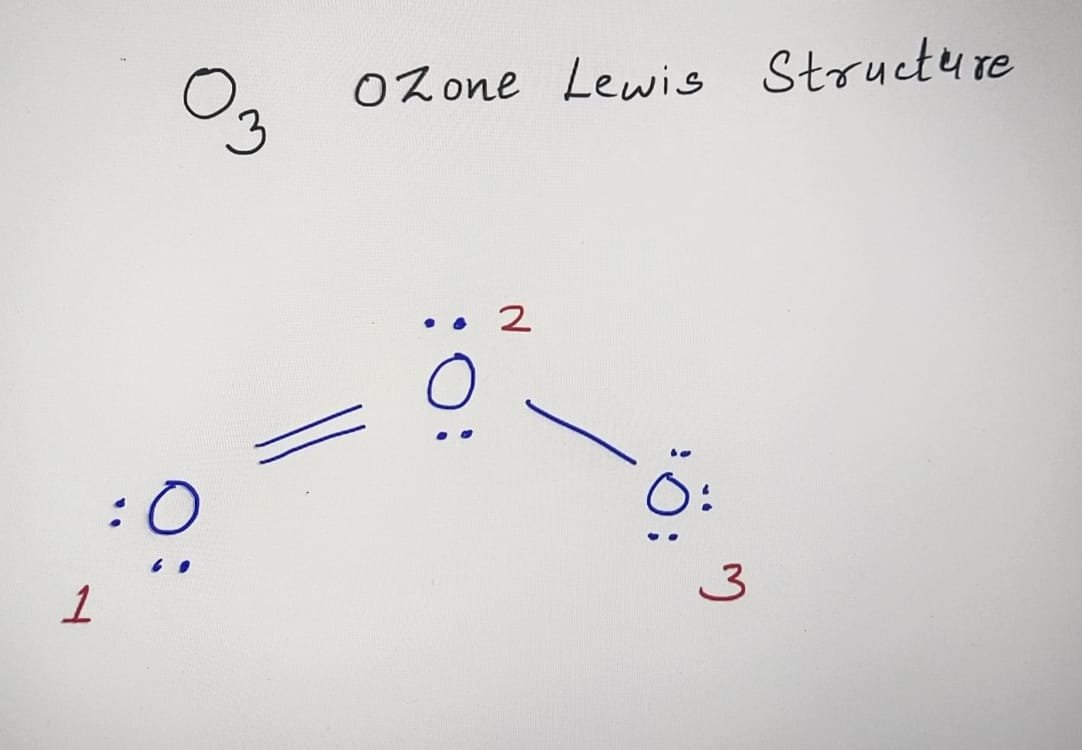
O3 Lewis Structure Formal Charge Basics of Chemistry
> P block elements Class 12 > O3 Lewis Structure Formal Charge O3 Lewis Structure Formal Charge. O3 Lewis Structure Formal Charge. February 23, 2023

confusion over resonance major contributor and reactivity r/Mcat
Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = 1+, which is the same as the total charge of the ammonium polyatomic ion. Exercise 2.3.1 2.3. 1. Write the formal charges on all atoms in BH−4 BH 4 −.

Formal charge on oxygen atoms marked as O1,O2 and O3 in O3 as per follow..
Formal charge on right Oxygen = Valence electrons - Nonbonding electrons - (Bonding electrons)/2 = 6 - 6 - (2/2) = 1-. So the formal charge on right oxygen atom is 1-. Now let's put all these charges on the lewis dot structure of O3. So there is overall 0 charge left on the entire molecule. This indicates that the O3 (Ozone) has 0 charge.
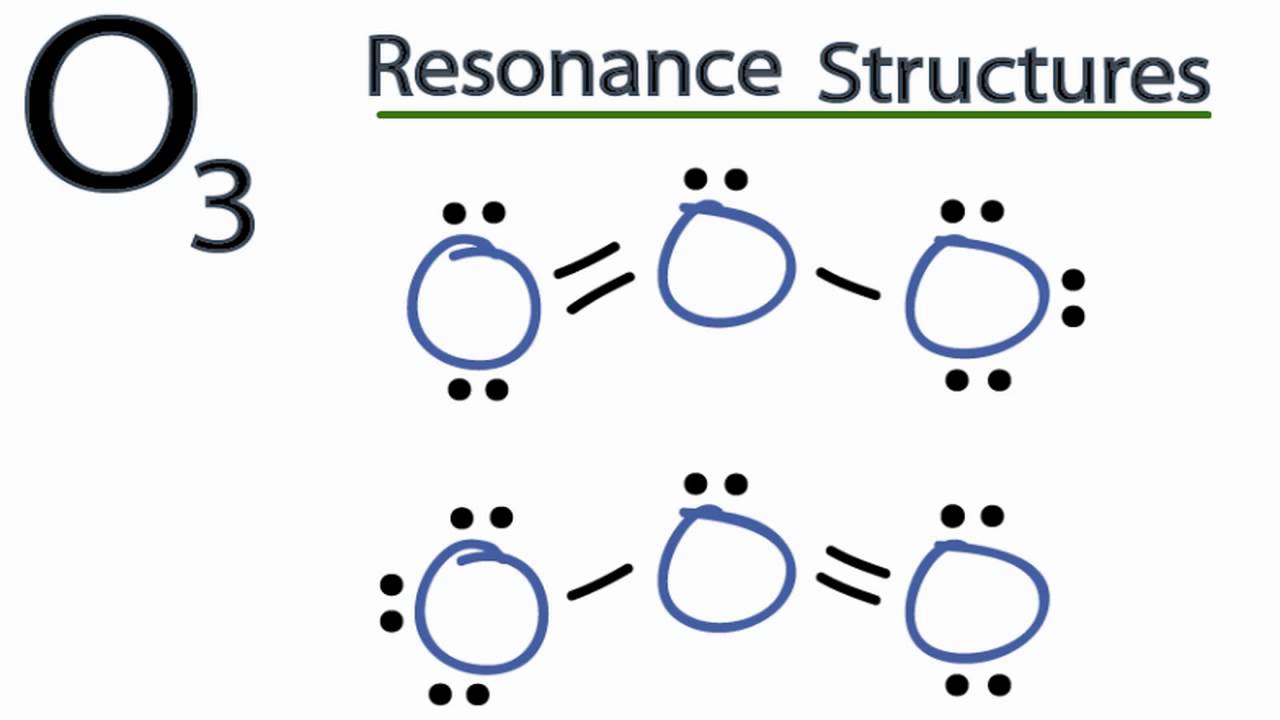
What are the formal charges in "O"_3 (ozone)? Socratic
Formal charges in ozone and the nitrate anion. In chemistry, a formal charge (F.C. or q*), in the covalent view of chemical bonding, is the hypothetical charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number of valence.

Calculating O3 Formal Charges Calculating Formal Charges for O3 (Ozone) YouTube
In the O3 Lewis structure, there is one double bond and one single bond around the oxygen atom, with two other oxygen atoms attached to it. The oxygen atom. Formal charge = valence electrons - nonbonding electrons - ½ bonding electrons. For left oxygen and right oxygen atom, formal charge = 6 - 6 - ½ (2) = -1.
Find the formal charge of ‘O’ in ozone.
When oxygen bonds we have found it to either have a formal charge of 0 (2 bonds and 2 lone pairs), +1 (3 bonds and 1 lone pair), and -1 (1 bond and 3 lone pairs). There are a couple other possibilities which you may run into when studying free radical reactions and such. Answer.
PPT Lewis structures and the geometry of molecules with a central atom. PowerPoint
The formal charge on O: 6-2-½ (6)= 1, thus the formal charge on o3 Lewis structure is +1 on the central oxygen atom. Similarly, two adjacent oxygen atoms carry (-½ ) partial negative charge, and central oxygen carries +1 formal charge as shown in the figure below. O3 lewis structure formal charges. By applying this formula, we find that each.
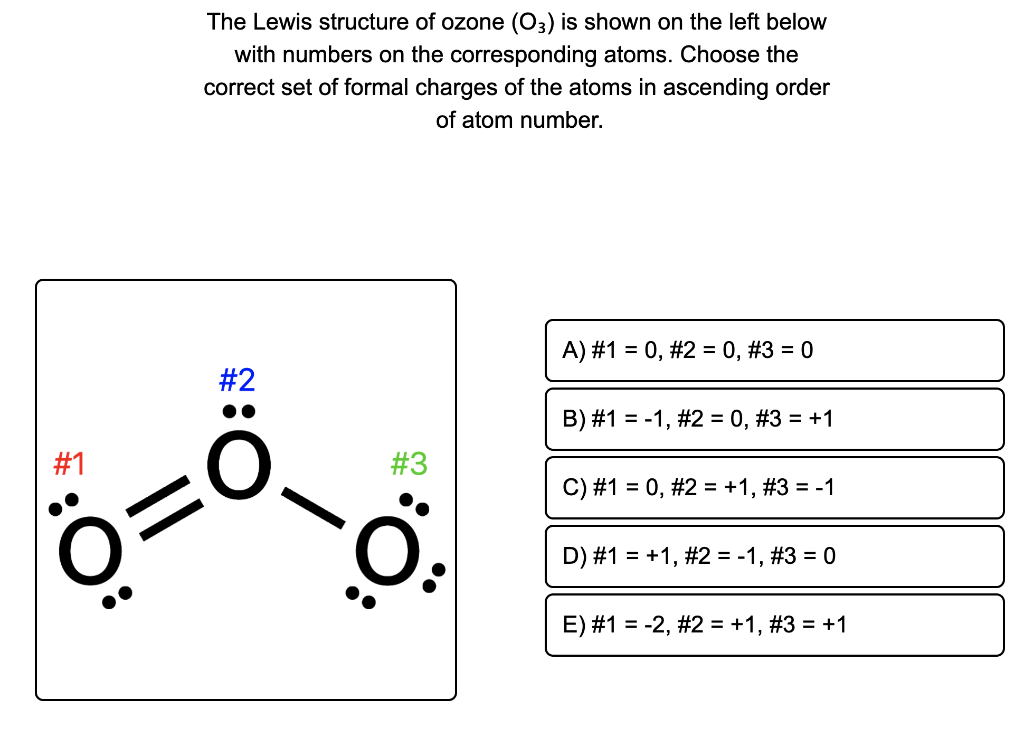
Solved The Lewis Structure Of Ozone (03) Is Shown On The
The formal charge of any atom in a molecule can be calculated by the following equation: FC = V − N − B 2 (1) (1) F C = V − N − B 2. where V is the number of valence electrons of the neutral atom in isolation (in its ground state); N is the number of non-bonding valence electrons on this atom in the molecule; and B is the total number.

O3 Lewis Structure Formal Charge Basics of Chemistry
Step 1. We divide the bonding electron pairs equally for all I-Cl bonds: Step 2. We assign lone pairs of electrons to their atoms. Each Cl atom now has seven electrons assigned to it, and the I atom has eight. Step 3. Subtract this number from the number of valence electrons for the neutral atom: I: 7 - 8 = -1.
no2 bond order
The correct option is B 1. Formal charge (F C) = V −L− B 2. Where, V = Total number of valence electrons in the atom. L = Total number of non bonding (lone pair) electrons in the atom. B = Total number of bonding (shared) electrons in that particular atom. Hence, the formal charge on the central O atom in O3 = 6−2− 1 2×6= +1. Suggest.

Calculating Formal Charge of O3 A StepbyStep Guide Actualizado October 2023
How to calculate the formal charges on the atoms of ozone (O3)MOC members get access to over 1500 quizzes on O3 and many other topics, plus Flashcards, the R.